Antiretroviral treatment in pregnancy has improved maternal health and dramatically reduced the rate of vertical HIV transmission. Over 1 million pregnant women take these medications annually. Yet, a public health challenge remains, identifying the safest antiretrovirals to optimize maternal-child health outcomes, while continuing to achieve high efficacy in preventing vertical HIV transmission. While antiretrovirals are essential for maternal health and preventing HIV transmission to the infant, antiretrovirals have been associated with increased risk for adverse birth outcomes including preterm and small for gestation age births. The mechanisms underlying these outcomes remain poorly understood.
With the overall objective of optimizing antiretroviral therapy for pregnant women with HIV and their infants, the aims of the Serghides Lab are: (1) to identify mechanisms that contribute to adverse birth outcomes in the context of HIV and antiretroviral exposure, as well as identify biomarkers of risk, and interventions to improve outcomes, (2) to understand the effects of in utero exposure to HIV and antiretrovirals on the development of children who are HIV exposed but uninfected and identify underlying mechanisms, and (3) to develop animal and ex-vivo models to facilitate the study HIV and antiretroviral effects in the context of pregnancy.
CURRENT PROJECTS

HIV, Antiretrovirals, and Hormonal Dysregulation in Pregnancy
Working with Canadian and international collaborators we are studying the impact of different classes of antiretrovirals on maternal hormones in pregnancy and how these changes affect the baby’s growth and development in utero and postnatally. Our lab was the first to identify a link between antiretroviral use in pregnancy and changes in progesterone and estradiol. This work is funded by a CIHR grant.
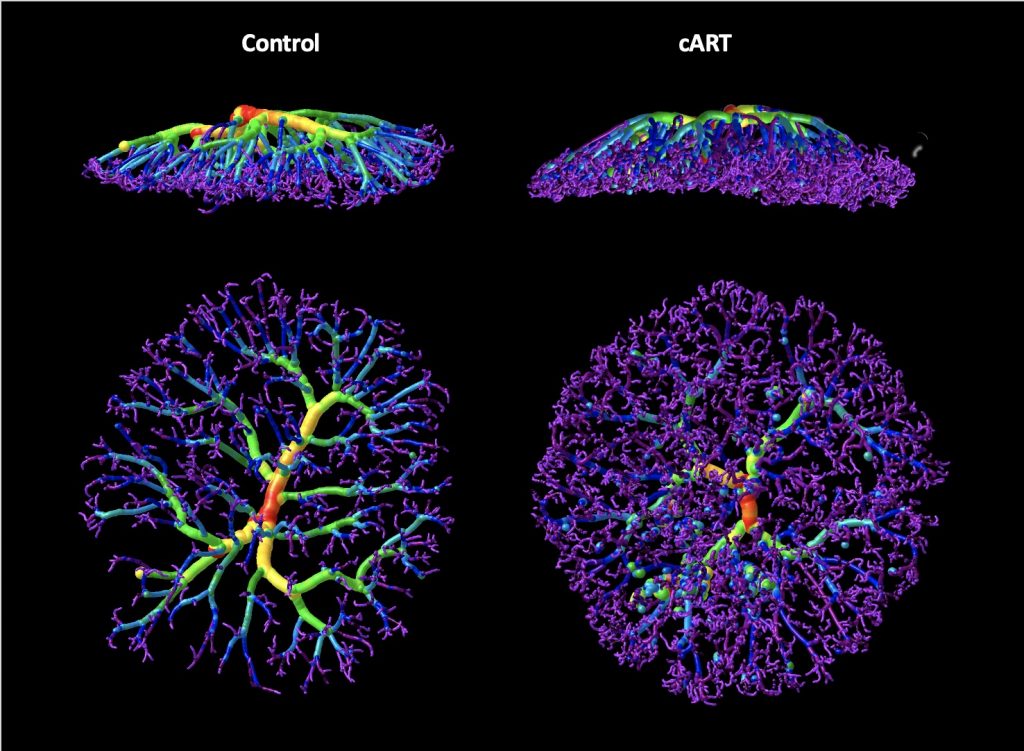
HIV, Antiretrovirals, and the Placenta
The placenta provides nutrients and oxygen, and protects the fetus from maternal immune allorecognition and from invasive pathogens. Placenta pathology has been reported in the context of HIV infection, but a gap in knowledge of the impact of different antiretrovirals on the placenta. Using human placenta samples from our own cohort and several international cohorts, together with an animal model we are examining the impact of different antiretrovirals on placenta development and structure, as well as metabolic and immune function. This work is funded by a CIHR bridge grant.
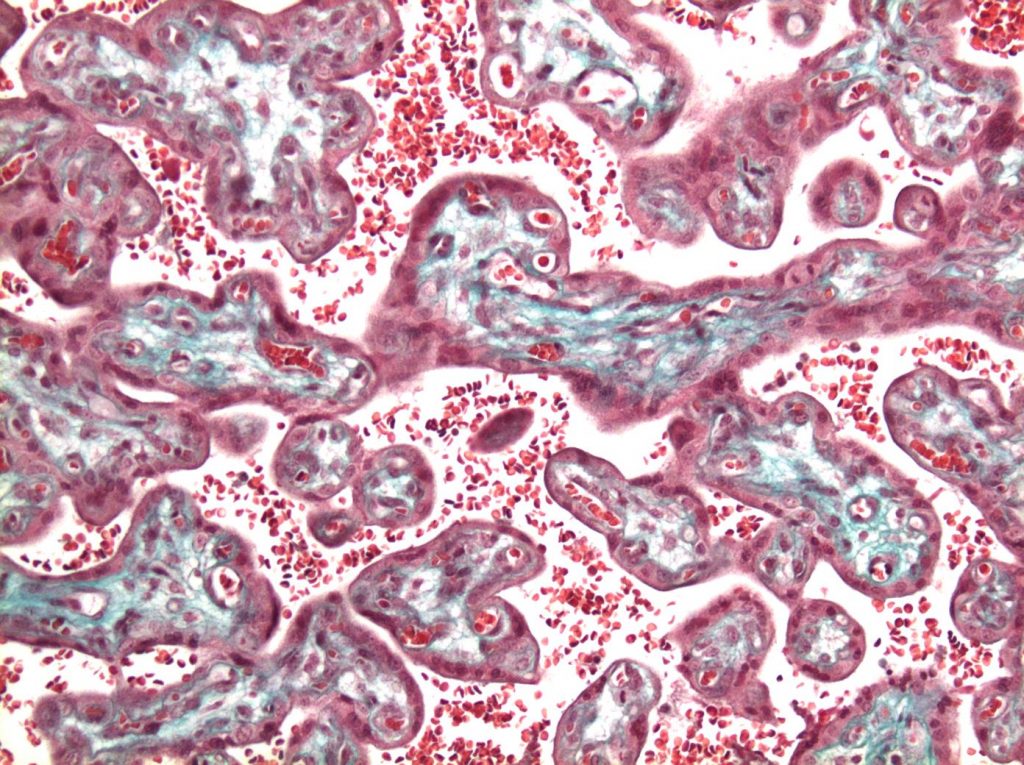
HIV, Antiretrovirals, and the Decidua
While early initiation of HIV treatment is recommended for people with HIV, exposure to antiretrovirals from conception may be associated with higher rates of poor birth outcomes. Utilizing an ex-vivo model using first trimester human decidual and placental tissue, as well as an animal model, we are studying the impact of different classes of antiretrovirals on early pregnancy events including decidualization, trophoblast migration, uterine immune cell function, and spiral artery remodeling. This work is funded by a CIHR project grant.

Impact of in utero Exposure to ARVs on Fetal Development and Neurocognitive Performance
Findings that the in utero environment is altered by HIV and antiretroviral exposure raises the question of long-term effects on the offspring. Using an animal model we are studying the impact of in utero exposure to antiretrovirals on neurodevelopment. With a large team of co-investigators, we are also investigating the impact of in utero HIV and antiretroviral exposure on behaviour, cognition, and brain structure in a cohort of children – the KIND cohort. This work is funded by a CIHR Team grant.
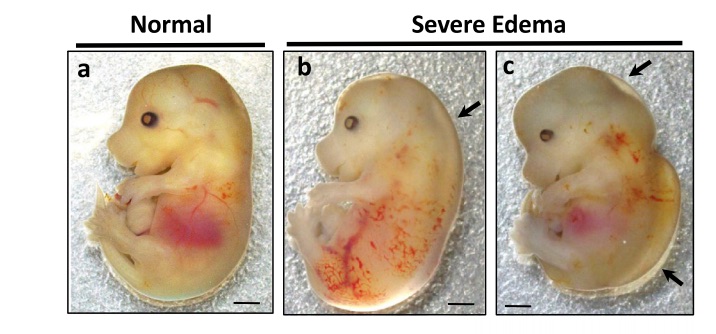
Dolutegravir in Pregnancy, Metabolic Alterations, and Fetal Defects
Treatment with dolutegravir from conception has been associated with increased risk for neural tube defects, which has created uncertainty over the use of dolutegravir in the treatment of women of reproductive age living with HIV. Dolutegravir has also been associated with excess weight gain, a known risk factor for neural tube defects, suggesting that dolutegravir-associated metabolic alterations could contribute to the increased risk for neural tube defects. Using animal models and clinical samples we are investigating the association between dolutegravir use in pregnancy and maternal and fetal metabolic alterations and the contribution to fetal defects. This work is funded by an NIH RO1 grant.
Thanks to Our Funders:
Our Host Institutions:
And the Institutions of our Collaborators and Partners:
(Logos and Trademarked Names held by respective institutions.)












